Atom
Di atom bi di baezik patikul of di kemikal elements. An atom konsis of a nukleus of protons and jeneraly neutrons, wey dey soraunded by an elektromagnetikaly-baund swarm of elektrons. Di kemikal elements dey diferenshiated from each oda bai di nomba of protons wey dey dia atoms. For exampul, eni atom wey kontain 11 protons bi sodium, and eni atom wey kontain 29 protons bi koppa. Atoms wey get di sem nomba of protons but a diferen nomba of neutrons bi isotopes of di sem element.
Atoms dey veri smol, typikally araund 100 pikometas akros. A human hair bi abaut a million kabon atoms waid. Dis dey smola than di shortes waivlength of visibul lait, which mean say humans no fit si atoms with konvenshonal maikroskops. Atoms dey so smol sotey to akuraetli predit their behavio bai yuzin klassikal fysiks no dey posibul due to quantum effekts.
Moh than 99.94% of an atom mass dey di nukleus. Protons get a positiv elektrik charj and neutrons no get charj, so di nukleus dey positivli charjd. Di elektrons dey negativli charjd, and dis oposin charj bi wetin dey baind dem to di nukleus. If di nombas of protons and elektrons dey equal, as dem normally dey, then di atom dey elektrikali neutral as a whol. If an atom get moh elektrons than protons, then e get an ovaraul negativ charj, and so bi a negativ ion (or anion). Konvasli, if get moh protons than elektrons, e get a positiv charj, and so bi a positiv ion (or kation).
Diskovri of di elektron
[chenj-am | chenj-am for orijin]For 1897, J. J. Thomson diskova say kathod rays no bi elektromagnetik waivs but konsis of patikuls bikos Dem fit dey deflekted bai elektrik and magnetik fields. Him measure diz patikuls to dey 1,800 taims laita than hydrojin (di laites atom). Thomson konklud say diz patikuls kom from di atoms wey dey di kathod—dem bi subatomik patikuls. Him kol diz niu patikuls kopuskuls but elektrons bi wetin wi kom to sabi dem as. Thomson also show say elektrons dey identikal to di patikuls wey dey komot from photoelektrik and radioaktiv materials.[1] Wi quickli rekognaiz say elektrons bi di patikuls wey kari elektrik korrents for metal waiya-dem.[2] Thomson konklud say diz elektrons join togeda from di veri atoms of di kathod for him instruments, wey mean say atoms no dey indivisibul as Dalton been tink.
Diskovri of di nukleus
[chenj-am | chenj-am for orijin]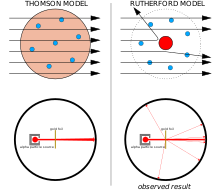
Left: results wey wi expekt: alfa patikuls wey dey pas thru di plum pudin model of di atom with neglijibul deflekshon.
Raight: Wetin wi obsav: koncentrated positiv charj of di nukleus deflekt a smol poshon of di patikuls.
J. J. Thomson been tink say di negativli-charjd elektrons dey distributed thruaut di atom for a sea of positiv charj wey dey distributed akros di whol volum of di atom.[3] Dis model bi wetin wi sometaims sabi as di plum pudin model.
Ernest Ruthaford and him koleagues Hans Geija and Ernest Marsden kom to daut di Thomson model afta dem encaunta difikultis wen dem trai to build instrument to measure di charj-to-mass ratio of alfa patikuls (diz bi positivli-charjd partikuls wey cetain radioactiv substansis such as radium dey emit). Di alfa patikuls dey skatad bai di air for di detekshon chamba, wey mek di measurements dey unreliabul. Thomson been enkaunta a simila problem for him work on kathod rays, wey him solv bai kreatin a nia-pafekt vakuum for him instruments. Ruthaford no tink say him ga run into dis sem problem bikos alfa patikuls dey much hevi than elektrons. Akordin to Thomson's model of di atom, di positiv charj for di atom no dey koncentrated enouf to prodius elektrik field wey ga dey strong enouf to deflekt an alfa patikul, and di elektrons dey so laitweight so dat dem ga dey push aside efotlesli bai di much hevi alfa partikuls. Yet skatarin dey, so Ruthaford and him kolleagues decide to investigaet dis skatarin kiafuli.[4]
Fission, hai-eneji fysiks and kondensd mata
[chenj-am | chenj-am for orijin]For 1938, di Jerman kemist Otto Hahn, a student of Ruthaford, direkt neutrons onto uranium atoms expektin say na transuranium elements ga bi di results. Insted, him kemikal experiments show barium as di produkt. A year afta, Lise Meitna and ha nefew Otto Frisch verifai say Hahn's result bi di fes experimental nukle fission. For 1944, Hahn receiv di Nobel Prize for Kemistri. Despite Hahn's efots, di kontribushons of Meitna and Frisch no dey rekognaizd.
For di 1950s, di development of impruvd patikul accelerators and patikul detetors kom allow saientists to stodi di impakts of atoms wey dey muv for hai enejis. Dem diskova say Neutrons and protons bi hadrons, or komposits of smola patikuls wey dem kol quarks.
Strukcho
[chenj-am | chenj-am for orijin]Subatomik partikuls
[chenj-am | chenj-am for orijin]Though di wod atom been orijinali mean patikul wey no fit dey cut into smola patikuls, for moden saientifik yuz di atom konsis of various subatomik patikuls. Di konstituent patikuls of a atom bi di elektron, di proton and di neutron.
Nukleus
[chenj-am | chenj-am for orijin]
Aul di baund protons and neutrons for an atom mek up a taini atomik nukleus, and dey kolektivli kol nukleons. Di radius of a nukleus dey approximaetli equal to femtometas, whe bi di total nomba of nukleons.[5] Dis dey much smola than di radius of du atom, wey dey 105 fm.
Atoms of di sem element get di sem nomba of protons, wey bi di atomik nomba. Within a singul element, di nomba of neutrons fit vari, to detamyn di isotop of dat element. Di total nomba of protons and neutrons detamyn di nuklyde. Di nomba of neutrons relativ to di protons dey detamyn di stabiliti of di nukleus, with certain isotops sufarin radioactiv dikay.
Propatis
[chenj-am | chenj-am for orijin]Nukle propatis
[chenj-am | chenj-am for orijin]Bai definishon, eni tuw atoms with an identikal nomba of protons for dia nuklei bilong to di sem kemikal element. Atoms with equal nomba of protons but a diferen nomba of neutrons dey diferen isotops of di sem element. For exampul, aul hydrojin atoms dey admit exaktli a proton, but isotops exist with no neutrons (hydrojin-1, bai fa di mos komon fom, wey bi also protium), a neutron (deuterium), tuw neutrons (tritium) and moh than tuw neutrons. Di elements wey wi sabi fom a set of atomik nomba, from di singul-proton element hydrojin up to di 118-proton element oganesson.
Mass
[chenj-am | chenj-am for orijin]Di laj majoriti of an atom's mass dey kom from di protons and neutrons wey mek up am. Di total nomba of diz patikuls (wey bi "nukleons") for a given atom bi di mass number. E bi a positiv intija and daimenshonles (instead of havin dimenshon of mass), bikos e dey expres a kaunt. An exampul of yuz of a mass nomba bi "kabon-12," wey get 12 nukleons (six protons and six neutrons).
Shayp and saiz
[chenj-am | chenj-am for orijin]Atoms dey lak a wel-defined auta baundari, so dia daimenshon yuzuali dey deskraibd in tems of an atomik radius. Dis bi a measure of di distans aut to which di elektron klaud dey extend from di nukleus.[6]
Wen e dey subjekted to extanal fosis, laik elektrikal fields, di shayp of an atom fit deviaet from sferikal symmetri.
Radioactiv dikay
[chenj-am | chenj-am for orijin]
Also si
[chenj-am | chenj-am for orijin]Notes
[chenj-am | chenj-am for orijin]Referens
[chenj-am | chenj-am for orijin]- ↑ cite journal|last=Thomson|first=J.J.|title=On bodi-dem wey dey smol pas atoms|journal=Di Popula Saiens Monthly|pages=323–335|date=August 19|url=https://books.google.com/books?id=3CMDAAAAMBAJ&pg=PA323%7Caccess-date=21 June 2009|archive-date=December 2016|archive-url=https://web.archive.org/web/20161201152039/https://books.google.com/books?id=3CMDAAAAMBAJ&pg=PA323%7Curl-status=live}}
- ↑ "Di Mekanisim Of kondukshon for Metals" url=https://web.archive.org/web/20121025004809/http://library.thinkquest.org/ |date=25 October 2012 , Think Quest.
- ↑ Navarro (2012). A Histori of di Elektron, p. 94
- ↑ Heilbron (2003). Ernest Ruthaford and di Explosion of Atoms, pp. 64–68
- ↑ Jevremovic, Tatjana (2005). Nukle Principuls for Engineering. Springer. p. 63. ISBN 978-0-387-23284-3. OCLC 228384008.
- ↑ Ghosh, D.C.; Biswas, R. (2002). "Theoretikal kalkulashon of Absolut Radii of Atoms and Ions. Part 1. Di Atomic Radii". Int. J. Mol. Sci. 3 (11): 87–113. doi:10.3390/i3020087.




![{\displaystyle 1.07{\sqrt[{3}]{A}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a74a6ca6998768195969eef75ca046e8431c29d3)
